Append a new shard of data#
We have one artifact in storage and are about to receive a new shard of data.
In this notebook, we’ll see how to manage the situation.
import lamindb as ln
import bionty as bt
import readfcs
bt.settings.organism = "human"
💡 connected lamindb: testuser1/test-facs
ln.transform.stem_uid = "SmQmhrhigFPL"
ln.transform.version = "0"
ln.track()
💡 notebook imports: anndata==0.9.2 bionty==0.42.4 lamindb==0.69.2 pytometry==0.1.4 readfcs==1.1.7 scanpy==1.10.0
💡 saved: Transform(uid='SmQmhrhigFPL6K79', name='Append a new shard of data', key='facs2', version='0', type=notebook, updated_at=2024-03-28 10:26:47 UTC, created_by_id=1)
💡 saved: Run(uid='sGhrGnLmOn9qloGNvS3K', transform_id=2, created_by_id=1)
Ingest a new artifact#
Access  #
#
Let us validate and register another .fcs file from Oetjen18:
filepath = readfcs.datasets.Oetjen18_t1()
adata = readfcs.read(filepath)
adata
AnnData object with n_obs × n_vars = 241552 × 20
var: 'n', 'channel', 'marker', '$PnR', '$PnB', '$PnE', '$PnV', '$PnG'
uns: 'meta'
Transform: normalize  #
#
import anndata as ad
import pytometry as pm
pm.pp.split_signal(adata, var_key="channel")
pm.pp.compensate(adata)
pm.tl.normalize_biExp(adata)
adata = adata[ # subset to rows that do not have nan values
adata.to_df().isna().sum(axis=1) == 0
]
adata.to_df().describe()
| CD95 | CD8 | CD27 | CXCR4 | CCR7 | LIVE/DEAD | CD4 | CD45RA | CD3 | CD49B | CD14/19 | CD69 | CD103 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| count | 241552.000000 | 241552.000000 | 241552.000000 | 241552.000000 | 241552.000000 | 241552.000000 | 241552.000000 | 241552.000000 | 241552.000000 | 241552.000000 | 241552.000000 | 241552.000000 | 241552.000000 |
| mean | 887.579860 | 1302.985717 | 1221.257257 | 877.533482 | 977.505533 | 1883.358298 | 556.687953 | 929.493316 | 941.166747 | 966.012244 | 1210.769935 | 741.523184 | 1003.064857 |
| std | 573.549695 | 827.850302 | 672.851319 | 411.966073 | 584.217139 | 932.113729 | 480.875917 | 795.550133 | 658.984751 | 456.437094 | 694.622980 | 473.287558 | 642.728024 |
| min | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |
| 25% | 462.757715 | 493.413744 | 605.463427 | 588.047798 | 495.437303 | 1063.670965 | 240.623098 | 404.087640 | 477.932659 | 592.294399 | 575.401173 | 380.247262 | 475.108131 |
| 50% | 774.350833 | 1207.624048 | 1110.367681 | 782.939692 | 782.981430 | 1951.855099 | 484.355203 | 557.904360 | 655.909639 | 800.280049 | 1124.574275 | 705.802991 | 775.101973 |
| 75% | 1327.792103 | 2036.849496 | 1721.730010 | 1070.479036 | 1453.929567 | 2623.975657 | 729.754419 | 1345.771633 | 1218.445208 | 1347.042403 | 1742.288464 | 1069.175380 | 1420.744291 |
| max | 4053.903716 | 4065.495666 | 4095.351322 | 4025.827267 | 3999.075551 | 4096.000000 | 4088.719985 | 3961.255364 | 3940.061146 | 4089.445928 | 3982.769373 | 3810.774988 | 4023.968008 |
Validate cell markers  #
#
Let’s see how many markers validate:
validated = bt.CellMarker.validate(adata.var.index)
❗ 9 terms (69.20%) are not validated for name: CD95, CXCR4, CCR7, LIVE/DEAD, CD4, CD49B, CD14/19, CD69, CD103
Let’s standardize and re-validate:
adata.var.index = bt.CellMarker.standardize(adata.var.index)
validated = bt.CellMarker.validate(adata.var.index)
❗ 7 terms (53.80%) are not validated for name: CD95, CXCR4, LIVE/DEAD, CD49B, CD14/19, CD69, CD103
Next, register non-validated markers from Bionty:
records = bt.CellMarker.from_values(adata.var.index[~validated])
ln.save(records)
❗ did not create CellMarker records for 2 non-validated names: 'CD14/19', 'LIVE/DEAD'
Manually create 1 marker:
bt.CellMarker(name="CD14/19").save()
❗ record with similar name exist! did you mean to load it?
| uid | synonyms | score | |
|---|---|---|---|
| name | |||
| Cd14 | 5JHfKNo5DC8y | 90.0 |
Move metadata to obs:
validated = bt.CellMarker.validate(adata.var.index)
adata.obs = adata[:, ~validated].to_df()
adata = adata[:, validated].copy()
❗ 1 term (7.70%) is not validated for name: LIVE/DEAD
Now all markers pass validation:
validated = bt.CellMarker.validate(adata.var.index)
assert all(validated)
Register  #
#
features = ln.Feature.lookup()
efs = bt.ExperimentalFactor.lookup()
organism = bt.Organism.lookup()
markers = bt.CellMarker.lookup()
artifact = ln.Artifact.from_anndata(
adata,
description="Oetjen18_t1"
)
Show code cell output
... storing '$PnR' as categorical
... storing '$PnE' as categorical
... storing '$PnV' as categorical
... storing '$PnG' as categorical
artifact.save()
artifact.features.add_from_anndata(var_field=bt.CellMarker.name)
❗ 1 term (100.00%) is not validated for name: LIVE/DEAD
❗ skip linking features to artifact in slot 'obs'
artifact.labels.add(efs.fluorescence_activated_cell_sorting, features.assay)
artifact.labels.add(organism.human, features.organism)
artifact.features
Features:
var: FeatureSet(uid='MnmcbnslK50LhCyQWgd3', n=12, type='number', registry='bionty.CellMarker', hash='YXolP9mtiV6-oHKhY4h6', updated_at=2024-03-28 10:26:51 UTC, created_by_id=1)
'Cd4', 'CD8', 'CD95', 'CXCR4', 'CD49B', 'CD69', 'CD3', 'CD103', 'CD27', 'CD14/19', 'Ccr7', 'CD45RA'
external: FeatureSet(uid='qUhpbDHohvEmzl7kkqo6', n=2, registry='core.Feature', hash='EcoLmu30NMuD7KX_L3Md', updated_at=2024-03-28 10:26:51 UTC, created_by_id=1)
🔗 assay (1, bionty.ExperimentalFactor): 'fluorescence-activated cell sorting'
🔗 organism (1, bionty.Organism): 'human'
View data flow:
artifact.view_lineage()
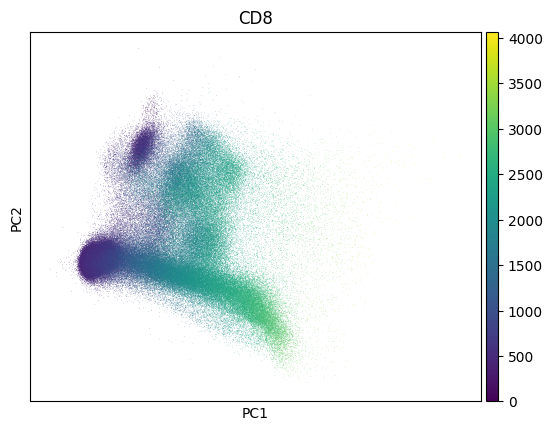
Inspect a PCA fo QC - this collection looks much like noise:
import scanpy as sc
sc.pp.pca(adata)
sc.pl.pca(adata, color=markers.cd8.name)
Create a new version of the collection by appending a artifact#
Query the old version:
collection_v1 = ln.Collection.filter(name="My versioned cytometry collection").one()
collection_v2 = ln.Collection(
[artifact, collection_v1.artifact], is_new_version_of=collection_v1, version="2"
)
collection_v2
Collection(uid='BXlEohVyuYed492l74D6', name='My versioned cytometry collection', version='2', hash='ZKQxIw0uAvtMtdZk8SAj', visibility=1, transform_id=2, run_id=2, created_by_id=1)
collection_v2.features
Features:
var: FeatureSet(uid='7cP0EbLEDtPXyUOr2n3S', n=41, type='number', registry='bionty.CellMarker', hash='n0jcZjyxOx4D0aylQKuM', created_by_id=1)
'CD57', 'Cd19', 'Cd4', 'CD8', 'Igd', 'CD85j', 'CD11c', 'CD16', 'CD3', 'CD38', 'CD27', 'CD11B', 'Cd14', 'Ccr6', 'CD94', 'CD86', 'CXCR5', 'CXCR3', 'Ccr7', 'CD45RA', ...
obs: FeatureSet(uid='EshIYFlw0mu7CUYg3Qxj', n=5, registry='core.Feature', hash='fn23ch8Df00zg3tBpZh_', updated_at=2024-03-28 10:26:41 UTC, created_by_id=1)
Time (number)
Cell_length (number)
Dead (number)
(Ba138)Dd (number)
Bead (number)
external: FeatureSet(uid='qUhpbDHohvEmzl7kkqo6', n=2, registry='core.Feature', hash='EcoLmu30NMuD7KX_L3Md', updated_at=2024-03-28 10:26:51 UTC, created_by_id=1)
🔗 assay (1, bionty.ExperimentalFactor): 'My versioned cytometry collection'
🔗 organism (1, bionty.Organism): 'My versioned cytometry collection'
collection_v2
Collection(uid='BXlEohVyuYed492l74D6', name='My versioned cytometry collection', version='2', hash='ZKQxIw0uAvtMtdZk8SAj', visibility=1, transform_id=2, run_id=2, created_by_id=1)
collection_v2.save()
collection_v2.labels.add(efs.fluorescence_activated_cell_sorting, features.assay)
collection_v2.labels.add(organism.human, features.organism)
collection_v2.view_lineage()


